Business Brief
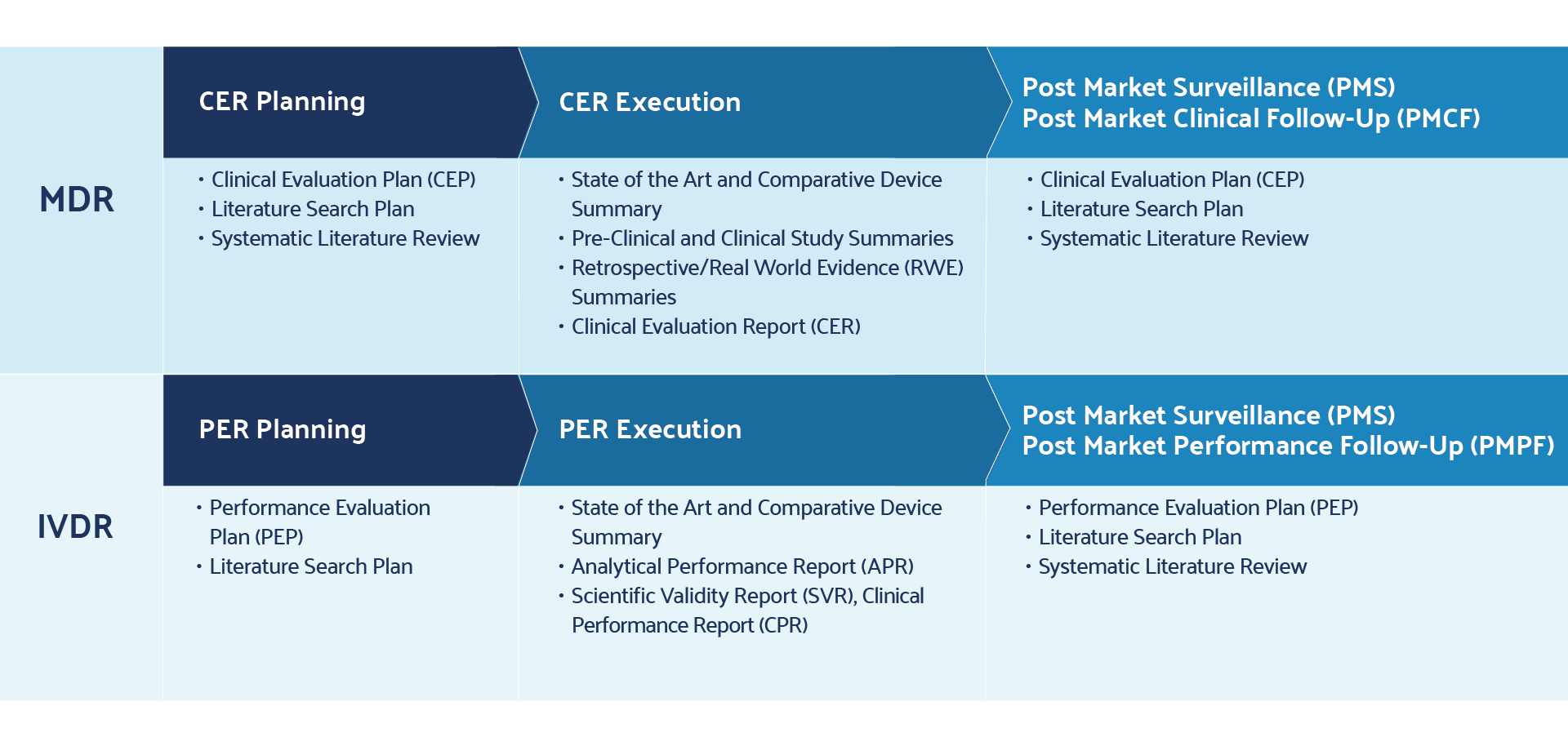
How Literature Review Automation Improves Clinical Evaluation Reports (CER) and Performance Evaluation Reports (PER) Program ManagementManufacturers in Europe and around the world are working to improve their compliance with the European Union’s Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR). For medical device and diagnostics manufacturers, doing business in the EU is now subject to increased regulatory oversight. Among other market challenges, these latest regulatory changes require companies to demonstrate clinical evidence, define state-of-the-art technology, and conduct post-market surveillance and post-market clinical and performance follow up on their devices to stay compliant with the medical device regulations.
With these additional regulatory requirements, state-of-the-art program management for product portfolios takes on an increasingly vital role for manufacturers submitting a Clinical Evaluation Report (CER) for the MDR program or a Performance Evaluation Report (PER) for the IVDR counterpart.
Unfortunately, traditional CER and PER program management, which often includes ad hoc management, inconsistently applied processes across different device product divisions, and the utilization of spreadsheets or non-purpose built software, are ill-equipped to meet more rigorous notified body and clinical evaluation report and performance evaluation report submission expectations – including the frequency and breadth of literature reviews for multi-product portfolios that require annual submissions.
The new medical device regulation also requires literature reviews of the existing clinical evidence informs the need for post-market clinical and performance follow-up studies. The topic of post market clinical evaluation will be covered in a subsequent business brief in this series.
Compounding the lack of a standard, automated approach for medical device literature reviews is the use of spreadsheets in their conduct. In fact, it is estimated that 90% of spreadsheets contain formula errors. These technical errors, coupled with the time and resources necessary to fix them, adversely impact the entire program management and clinical evaluation process. That leads to delays, omitted references, mistakes – or, worse, rejected clinical evaluation report submissions.
Implementing automation in medical device literature reviews, however, can help firms take control of the clinical evaluation program management process. Beyond simply allowing for broader yet more efficient searches, leveraging software to automate literature reviews can organize references, assign screeners, and review screening decisions. This saves time, reduces bottlenecks, and, most importantly, leads to a highly transparent, standardized, and repeatable process that supports continuous CER and PER submissions across a product portfolio and for the life of a device.
Understanding the Value of Literature Reviews in Improving CERs and PERs in Clinical Evaluation
Before exploring how to automate literature reviews for clinical evaluation reports and performance evaluation reports, it is important to first understand what are clinical evaluation reports and what a literature review entails. A literature review is a rigorous and robust method of evidence-based research used to collect, review, and report on large sets of published data in order to answer a well-defined research question.
Unlike a primary study, which collects data from an original source through methods such as a survey, randomized clinical trial, or case study, a literature review focuses on the entire catalog of data about a specific topic. Along with published scientific papers and studies, this data can include so-called gray literature such as white papers, unpublished manuscripts, and conference presentations.
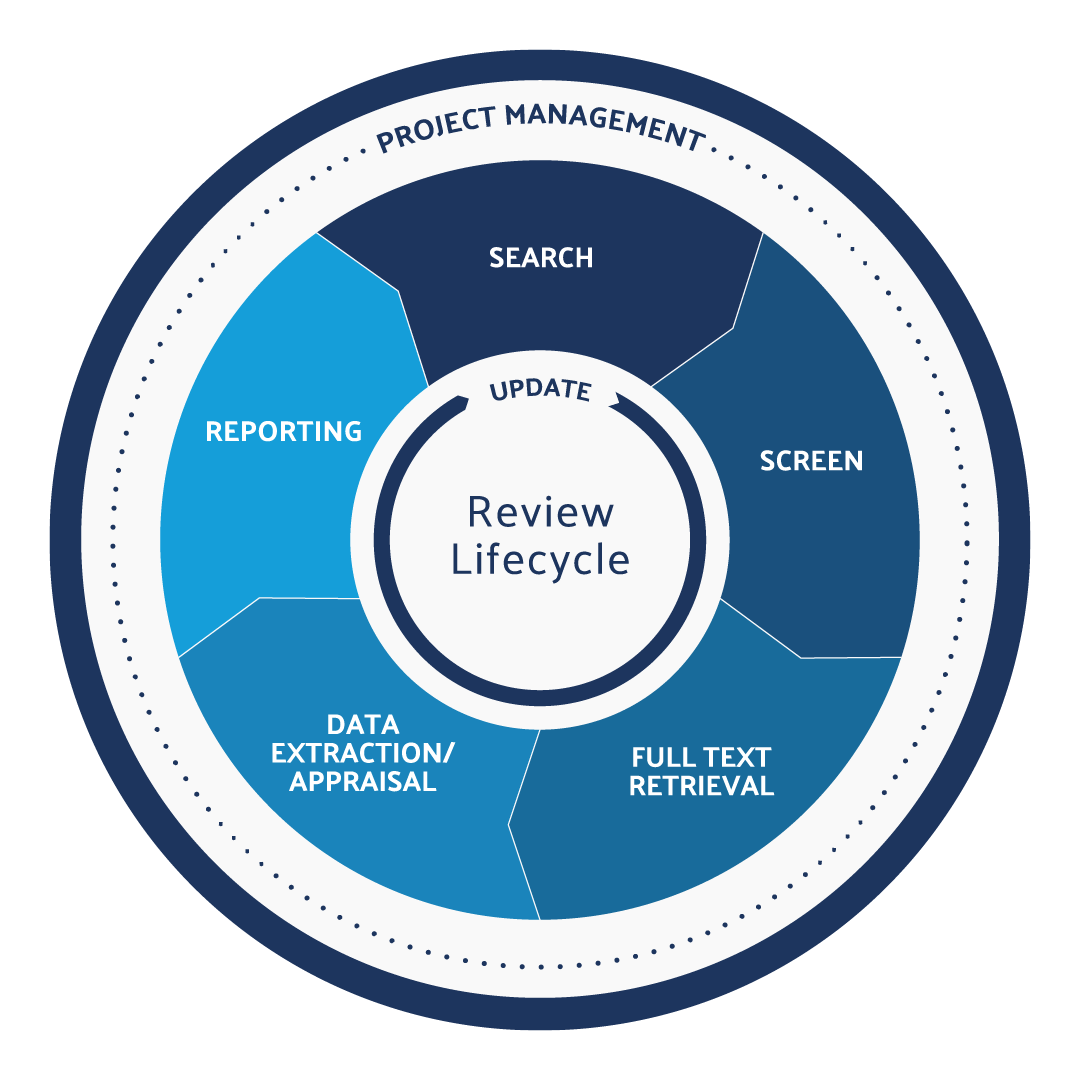
The lifecycle of the literature review process consists of six key steps:
- Define the research question.
- Search relevant information databases.
- Screen references for relevance.
- Full-text retrieval.
- Extract and appraise the data’s quality.
- Document purpose, methods, results, and conclusions.
- Monitor for new content and adjust the research question if necessary.
All literature reviews aim to achieve the goals of efficiency, transparency, and reproducibility. Best practices such as dual independent screening, effective collaboration, wide-ranging searches, well-defined inclusion and exclusion criteria, and well-documented lists of excluded references are critical in achieving those goals. In figure 2, literature reviews are a critical backbone to the PER and CER planning process, report development, post-market surveillance, and follow-up.
By automating literature reviews, medical device companies gain greater visibility throughout the enterprise into the management, quality, and cost of their notified body submissions – greatly enhancing the likely approval of their clinical evaluation reports and performance evaluation reports and devices.
Three Reasons Automated Literature Reviews Matter for Medical Device Manufacturers
The literature review process is not without its challenges. Large datasets require significant effort and collaboration to be screened properly, but few research teams have the required resources to support this process effectively.
Additionally, stringent MDR and IVDR regulatory requirements put more pressure on medical device manufacturers to show transparent, systematic, and reproducible review processes; this increased workload is difficult to manage using ad hoc processes. This can lead to a rushed review that’s increasingly susceptible to human error and likely to be rejected by a notified body, which can result in lost revenue as a result of time to market delays.
Automating the literature review process, though, brings three important benefits to medical device companies preparing CER and PER submissions.
Do More Faster and Smarter
MDR and IVDR regulatory requirements force medical device manufacturers to increase the frequency, traceability, and overall documentation of clinical and performance evaluation reports. This presents a number of specific challenges to research teams: a heavy documentation burden, ongoing monitoring for literature updates, continual updates to research libraries and other relevant project information, and an increased likelihood of user, bias, and conflict among reviews as the volume of literature increases.
All of these challenges lengthen the time necessary to complete a quality literature review and clinical evaluation. That is the last thing research teams need when reviews are both more frequent and more thorough. Automating the literature review process accelerates screening of references, provides easier access to source material, and immediately flags disagreements among reviewers that require resolution.
With automation in place, research teams can complete more accurate literature reviews in less time. For example, DistillerSR works with one firm where automation has reduced CER literature review times by 30% as compared to reviews managed in an ad hoc manner with spreadsheets. Another firm has greatly reduced reference screening times for titles and abstracts by more than 70% and cut time for the creation of diagrams and flowcharts by more than 50%, all of which has translated into a seven-figure cost savings for the company.
Take Control of the Clinical Evaluation Submission Process
One manufacturer DistillerSR worked with previously had a literature review process that was 100% manual. A medical writer would request articles from the library, obtain the abstracts, read and highlight the abstracts in a paper document, copy and paste the information into a separate document, go back to the library to retrieve the full-text articles, and then set up inclusion and exclusion rules in a spreadsheet. This may be an extreme example of completing a literature review, but many medical device manufacturers have similar processes in place for literature reviews used in clinical evaluation reports and performance evaluation reports. This type of approach poses numerous problems. It is unable to scale beyond a single submission; it won’t stand up to the scrutiny of the notified body; and it varies based on the experience and industry knowledge of the medical writers.
To stand up to the requirements of CER and PER submissions, the literature review process needs to be configurable. Steps in this direction include review templates that adjust to meet the needs of a specific notified body, cloud-based project hosting that enables access for the entire team regardless of location, and seamless connections to corporate libraries. Manual literature reviews for CER and PER submissions cannot support this level of configuration, but literature review software is positioned to do just that.
A configurable review process, then, is structured, standardized, and repeatable. It is capable of supporting continuous literature reviews for ongoing CER and PER submissions – some of which must be resubmitted annually for high-risk devices – and a broader device portfolio across the company. But the biggest benefit is time: Research teams spend less time completing logistical tasks such as data entry and more time using their expertise to complete a thorough literature review.
Manage a Single Source of Trusted Evidence
Spreadsheet-based processes lead to more than just inefficiency. Inconsistent manual processes for sharing, distributing, and tracking information increase the likelihood of errors, whether through incorrect formulas or copy-and-paste mistakes. In addition, these inconsistencies hinder gap analyses. With limited insight, gaps cannot be identified until the later stages of a review, which could require the revision of a research question. Altogether, this impacts the quality of the literature review – which can lead to rejected submissions and costly delays.
Automation addresses these issues by allowing research teams to track all review activity. This provides real-time insight into progress of the literature review as a whole, and it enables quality control measures such as double-checking and verifying exclusions. The ability to capture and validate data as reviewers enter it also provides valuable benefits, as it associates every action with the individual who affected it while removing the need for time-consuming data cleansing.
Ultimately, workflows are secure and contained within a single project, and the entire process is transparent to all participants – making it both defensible and auditable. A single source of truth also provides better insight into potential gaps and resourcing needs than an ad hoc, spreadsheet-driven process; this insight has the added benefit of creating a more informed and visible pipeline for literature reviews across a product portfolio or business unit.
Literature Review Automation Modernizes CER and PER Program Management
The most immediate impact of automated literature reviews for clinical evaluation reports and performance evaluation reports is improved efficiency, as research teams spend less time fixing preventable mistakes. However, the true impact extends further. Automated reviews enable a more transparent, repeatable, and auditable process, which allows manufacturers to create and implement a standard framework for literature reviews that can then be inserted into all CER and PER program management plans consistently across every device product, division, and research groups.
With a repeatable process in place, program management can focus less on the nuance of literature reviews and more on critical management and communication tasks that help move a clinical or performance evaluation program along. In addition, a familiar process means fewer gaps and faster identification of the gaps that do exist, which reduces bottlenecks and delays. Standard workflows for all literature reviews, meanwhile, enable new team members to contribute proactively and quickly across portfolios, reducing training times and establishing a consistent CER and PER process. Finally, transparency and repeatability support a continuous submission process – which is critical for annual recertification of high-risk devices in the market and ongoing program managing for devices across multiple portfolios.
Traditional program management is ill-suited for the literature review process now mandated for ongoing compliance with the European Union’s MDR and IVDR regulatory regulations. Implementing an automated literature review allows for faster and more accurate reviews while enabling the creation of a process-driven framework that can be applied to all future literature reviews. For manufacturers struggling to manage their pipeline of CER and PER submissions, an automated literature review process can bring much-needed order and set the stage for program management success.
Download the PDF version of this solution brief.
Related Resources
Blog
How DistillerSR has empowered market leaders Philips and Alcon to achieve faster and more accurate literature reviews for CER submissions.

Case Study
Dr. Bonnie H. Weiner describes the way DistillerSR reduced the time it took to complete CER literature reviews by approximately 30%.

Blog
Developing a repeatable, transparent process will lead to streamlined, audit-ready, and compliant literature reviews for your PER submissions.


