Post-Market Surveillance
Medical Device Guidance

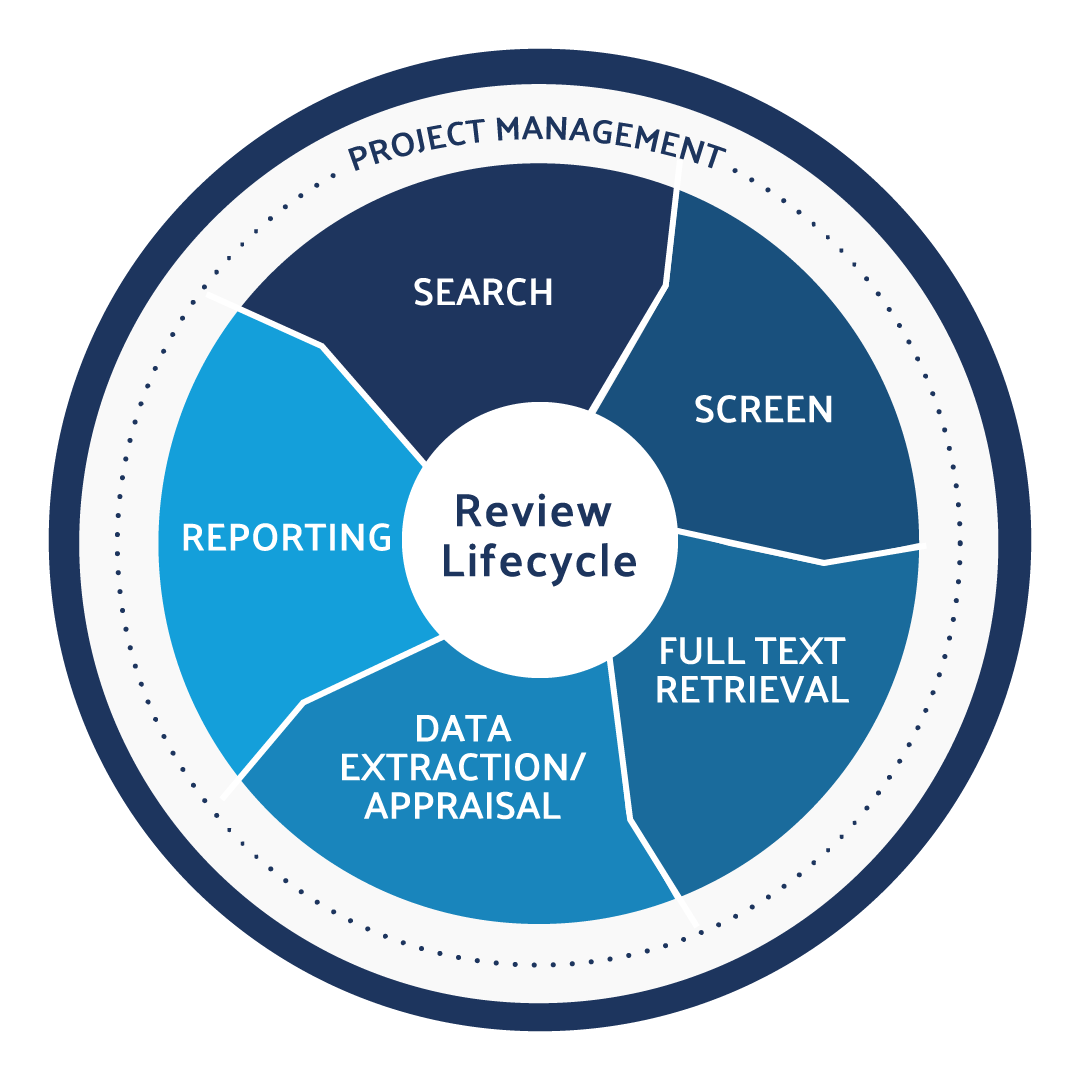
Use DistillerSR to produce CER and PER literature reviews in an efficient, audit-ready, and compliant way.
Traditionally, PMS relies on reactive data gathering in which manufacturers collect and report adverse events from a device. These adverse events are reported to the manufacturer or regulatory bodies, such as the FDA in the US, MHRA in the UK, and Health Canada in Canada. Another authority supporting manufacturers as they pursue EU-MDR compliance, is the Medical Device Coordination Group. This group is responsible for providing guidelines, recommendations, clarification of positions and topic overviews, such as this example exploring vigilance terminology and concepts in the MDR.
Manufacturers must also investigate products on the market, including active medical device reporting (MDR) and determine how best to inform regulatory authorities within a specific time frame if the issue is related to the device safety and performance. Manufacturers must re-evaluate the device’s benefit/risk ratio and safety or effectiveness in the post-market phase throughout this process. .
What Is Post-Market Surveillance for Medical Devices?
PMS activities are a critical aspect of pharmacovigilance for pharmaceutical products such as drugs, and retaining product vigilance for medical devices on the market. PMS allows for collecting data and information from a larger population, providing a more comprehensive understanding of a device’s safety and effectiveness than what can be obtained from clinical trials. The real-world clinical data and performance-based evidence from PMS activities allow manufacturers to continuously evaluate a device’s performance.
PMS requires gathering data on a device’s quality, safety, and performance throughout its life to continuously evaluate a device’s risk-benefit profile. This analysis also includes reviewing data on similar devices from competitors. Post-market data collection is vital for maintaining the safety and efficacy of medical devices, as well as providing R&D teams with much needed voice of customer feedback which can inform product refinement during the iterative design process. PMS activities include internal vigilance, where manufacturers gather customer complaints, failure analysis, adverse event reports, and external vigilance.
Articles 61 and 62 of the MDR outline how manufacturers can continually assess the risk/benefit ratio of their devices. Further guidance can be sought from ISO 14791. In essence, pharmaceutical and medical device producers rely on results from PMS to:
Identify potential hazards through pharmaco- and device vigilance, respectively
- Compare device performance to existing standards
- Comply with regulatory post-market surveillance mandates
- Continuously evaluate safety and effectiveness in real-world use
How Is Post-Market Surveillance Conducted?
At the top level, manufacturers are required to take these steps to perform PMS:
- Create a PMS strategy that includes determining the need for post-market clinical (PMCF) follow-up
- Execute the strategy
- Prepare PMS reports based on the collected data
A PMS plan lays out a manufacturer’s strategy for regularly monitoring and collecting data and safety information on its device. It’s an essential part of the device’s technical documentation, which also includes criteria for evaluating the device’s risk/benefits assessment and processes for:
- Compiling and analyzing data
- Following up on received complaints
- Communicating information to authorities and end-users
- Implementing corrective measures on devices
- Developing a PMCF plan or justifying reasons for not requiring one
The reporting requirements are different depending on the region, as shown below. Generally, reports include data analysis and an explanation of the actions taken to correct and prevent the issue.
- In the U.S., pharmaceutical manufacturers must file a Periodic Adverse Drug Experience Report (PADER).
- In the EU, manufacturers of Class I medical devices which are low-risk must develop a post-market surveillance report (PMSR). Manufacturers of Class IIa, Class IIb, and Class III devices must file a periodic safety update report (PSUR).
As part of a device’s technical documentation, these reports must be regularly updated according to the relevant regulatory bodies’ schedules.
Learn More About DistillerSR
(Article continues below)
What Are Post-Market Surveillance Activities?
Post-market surveillance activities are a set of activities performed by manufacturers. They include collecting and evaluating data on medical devices actively being used on the market.
This is a vital tool for ensuring medical devices continue to be safe and perform well and for taking action if the risk of continued use outweighs the benefit. The evaluation of post-market surveillance can also reveal opportunities for continuously improving medical devices.