Post-Market Surveillance Plan
Template for the MDR

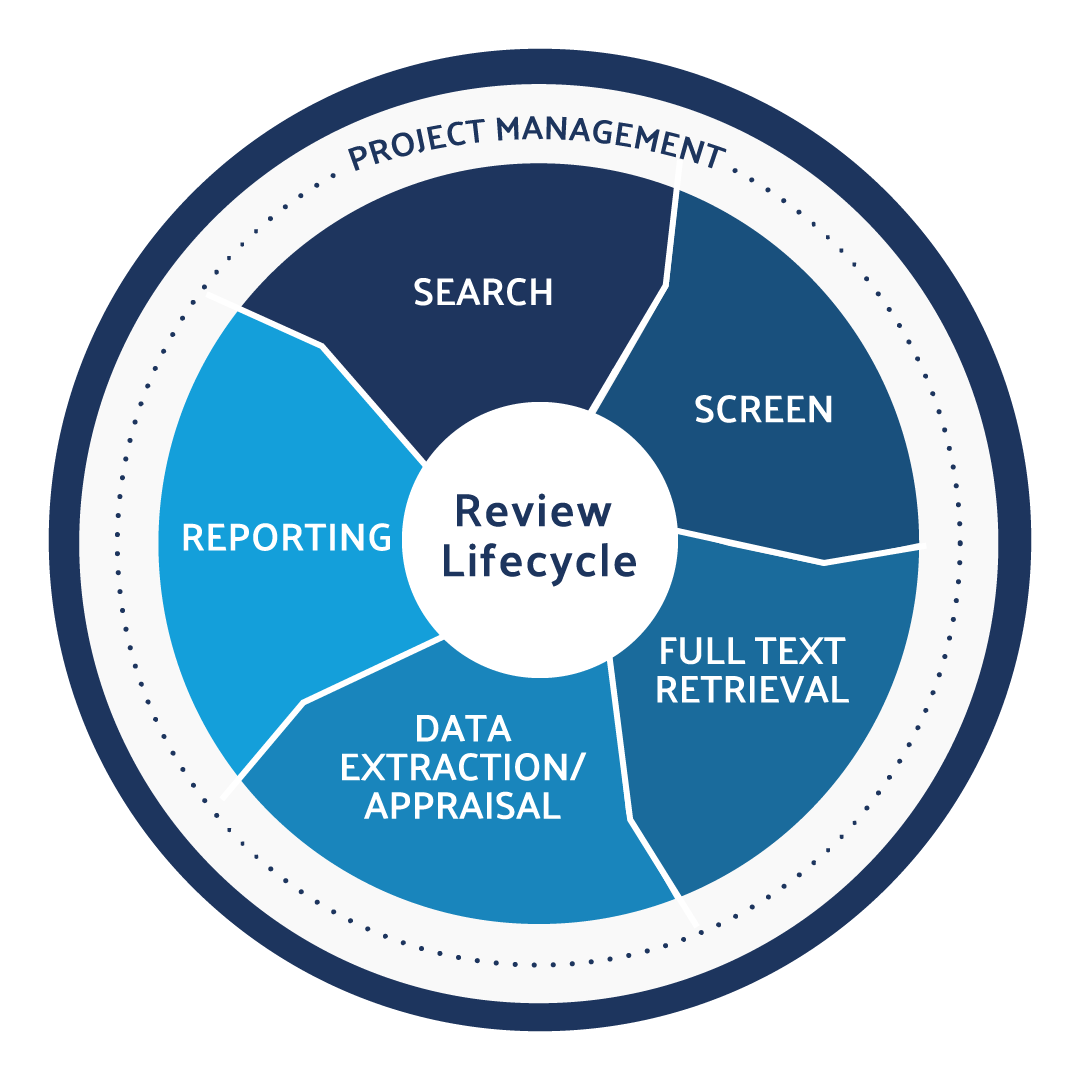
Use DistillerSR to produce CER and PER literature reviews in an efficient, audit-ready, and compliant way.
The medical device industry is a highly regulated business sector that requires vigilant surveillance to ensure the safety and efficacy of its devices. Post-market surveillance (PMS) is a critical component of this surveillance, as it allows manufacturers to collect meaningful feedback from their users and identify any potential issues with their products.
A comprehensive and detailed PMS plan is essential to ensuring that high-quality data is collected. The PMS plan requirements are laid out in Article 84 and Annex III of the MDR. The PMS template provides a structured approach to setting up a PMS process, defining the responsibilities of each team member, and outlining how feedback will be gathered and analyzed.
With a template, companies can create an effective plan to ensure accurate PMS.
PMS Templates Explained
PMS templates are a tool used to help manufacturers create PMS plans that comply with the European Medical Device Regulation (MDR). The templates outline the steps needed to create an effective and comprehensive plan. The MDR replaced the Medical Device Directive (MDD) in May 2021.
A post-market surveillance medical device template includes the following components:
Definition of Roles and Responsibilities
Identifying and assigning roles and responsibilities is key to successfully implementing PMS. This section assigns duties to each team member, outlining who is responsible for each aspect of the PMS process.
Data Collection Methods
This section outlines how you’ll collect data and the types of data to be collected, such as customer feedback or technical information. It should also detail the methods used to gather this data, such as user surveys, product registries, post-market clinical follow-up (PMCF), and complaint systems.
You might wonder what PMCF in the MDR may be. PMCF is a key component of the MDR, as outlined in Annex XIV, Part B. Manufacturers should continuously = review PMCF data and proactively report results to the relevant national regulatory bodies.
Analysis of Collected Data
Once data has been collected, it must be analyzed to identify any potential issues or trends. This section should outline the methods used to analyze the data and the criteria for determining whether a potential issue needs to be addressed.
Escalation and Corrective Action Processes
If potential issues are identified, this section should explain the procedures for escalating and responding to them. It should also detail any corrective or preventive actions that need to be taken.
Protocols for Document Management
PMS plans must be regularly updated and stored in a secure system. This section outlines the protocols for managing documents, including creating backup copies and ensuring that only authorized personnel have access.
Communication Strategies
Effective communication is essential for the successful implementation of PMS plans. This section should outline the strategies to communicate with stakeholders and keep them informed about the PMS process and results.
Audit and Review Process
The audit and review process is key to ensuring that the PMS plan is up-to-date and compliant with regulations. This section should explain how the plan will be audited and reviewed and any corrective actions that may need to be taken if noncompliance is identified.
Training Procedures
It is important that all stakeholders, including employees, understand their roles and responsibilities in the PMS process. This section should outline the training procedures that will ensure that everyone is adequately prepared. Impact to other SOP’s and procedures can be noted in this section.
Manufacturers can utilize the PMS plan template to develop comprehensive plans in compliance with MDR regulations, enabling them to review customer feedback and improve their products. While post-market surveillance for the MDD is only mandatory for Class III medical devices, all manufacturers need to contemplate the significance of PMS and take steps towards guaranteeing that their items are secure and functional.
Benefits of Using a PMS Plan Template
Here are some of the main benefits of using a PMS template:
1. Standardizes PMS Processes
A PMS plan template provides a standard approach to setting up and running PMS programs. This allows manufacturers to ensure that their PMS processes are consistent and compliant across all products. The result is a higher success rate as maintaining compliance with MDR, including fewer audit findings during regulatory reviews.
2. Streamlines Data Collection
The PMS plan template also helps to streamline the data collection process. By establishing methods for collecting and analyzing data, manufacturers can quickly identify potential product issues.
3. Improves Product Quality
By collecting customer feedback, manufacturers can use the data to improve their products. With a comprehensive PMS plan in place, companies can quickly identify areas for improvement and make changes as needed.
4. Enhances Customer Satisfaction
Finally, a PMS template can help to enhance customer satisfaction. By actively monitoring customer feedback and responding to any complaints or issues, manufacturers demonstrate to their customers that they value their opinions and are committed to providing high-quality products.
Learn More About DistillerSR
(Article continues below)
In Conclusion
The PMS plan template is an essential tool for any manufacturer looking to ensure that their products meet the highest safety standards. By providing a standardized approach to data collection, the MDR helps manufacturers reduce compliance risk and ensure that their products remain safe and of the utmost quality.